Part 2: From Lab to Market: Practical Pathways for Australia-China Collaboration
Introduction
In Part 1 of this series, we explored why China's biotech ecosystem has reached a level of sophistication that Australian companies can no longer afford to ignore—and why Australia's complementary strengths in research, clinical trials, and regulatory credibility create natural partnership opportunities.
But ecosystem-level observations only take you so far. The real question is: How do these partnerships actually work in practice?
The panel discussion at the Australia-China Bioinnovation Roundtable brought together leaders with direct, hands-on experience navigating cross-border collaboration—from conducting clinical trials to spinning out university IP to building integrated drug discovery platforms. Their insights moved the conversation from strategic possibility to operational reality.
The Panel: Collaboration Beyond Borders
The panel discussion, moderated by Betty Zhang, Investment Manager at Boson Ventures, brought together diverse perspectives representing clinical research, university technology transfer, policy research, and industry operations. The panelists included Dr. Dishan Herath (Chief Medical Officer, Peter MacCallum Cancer Centre), Maria Halasz (Head of New Ventures, UNSW; Director, Garvan Research Foundation), Dr. Marina Zhang (Associate Professor, Australia-China Relations Institute, University of Technology Sydney), and Dr. Xian Bu (Chief Business Officer & Executive Vice President, Viva Biotech).
Clinical Research Quality and Governance
Australia's expertise in first-in-human studies and regulatory excellence complements China's large patient populations and efficient clinical infrastructure, creating natural synergies for international collaboration built on rigorous standards.
Dr. Dishan Herath emphasized the importance of combining these strengths while maintaining clear governance frameworks, data quality assurance, and transparent communication. He highlighted Peter Mac's selective approach: the hospital accepts only 20% of trials offered, focusing on protocol quality and CRO selection that meets global standards. Peter Mac has already established strong partnerships with Chinese biotechs, particularly BeiGene (now BeOne Medicines), where Australian clinicians played crucial roles in early development.

Dr. Marina Zhang provided context on China's capital-efficient clinical trial landscape, noting that Phase III costs average $25,000 USD per patient—roughly one-third of US costs—with dramatically faster recruitment timelines.Betty Zhang reinforced these observations with insights from her recent China visit, where leading US investors expressed particular enthusiasm about China's clinical trials capability. They pointed to the compelling combination of speed, scale, and increasingly sophisticated trial design capabilities—enabling rapid iteration and innovation of Chinese drug developers to progress molecules to clinic.

Technology Transfer and Commercialization
Universities play a vital role in innovation ecosystems as bridges between fundamental research and commercial application. Australian institutions have evolved from traditional technology transfer models focused on IP licensing toward more sophisticated approaches that include equity investments, incubator programs, and direct spin-out support—enabling researchers to translate discoveries into viable companies while maintaining academic excellence.
Dr. Marina Zhang emphasized that institutional frameworks and government support are crucial enablers. Maria outlined UNSW's approach: as one of Australia's most entrepreneurial universities (voted top for four years), institutional commitment matters. UNSW provides up to $100,000 for early validation and $500,000 in pre-seed funding to prepare spin-outs for professional investors, including those in China.UNSW's formal partnership with China's Ministry of Science and Technology (established 2016) has delivered approximately $140 million in contract research, with physical presence in Wuxi demonstrating how institutions can engage deeply with proper frameworks.
Leveraging China's Integrated CRO/CDMO Capabilities
Effective execution leverages each ecosystem's strengths: China's world-class CRO capabilities and lower costs accelerate development, while Australia's IP protection and regulatory credibility provide global market validation.
Dr. Xian Bu brought unique perspective—having co-founded SYNthesis Med Chem in Melbourne in 2007, building it into a leading medicinal chemistry CRO, and ultimately selling to Viva Biotech in 2021. He traced the evolution of China's service ecosystem:
Ten years ago, there were many niche CROs, but today most have evolved into integrated platforms offering everything from protein production and structural biology through medicinal chemistry, bioassays, and ultimately CDMO manufacturing—all in one shop.

This integration creates significant advantages: reduced friction between vendors, better communication across the drug discovery workflow, cost efficiency, and accelerated timelines. Dr. Bu was direct in his assessment: the rest of the world, especially America, is taking advantage of China's speed and scale. Australia should take even more aggressive steps forward rather than following cautiously. Dr. Bu emphasised clear IP agreements, transparent communication, and cultural respect as essential elements of successful collaboration with service providers.
💡Key Lessons from Cross-Border Integration
The acquisition of SYNthesis Med Chem by Viva Biotech exemplifies strategic cross-border collaboration built on several key principles:
- Relationship-first approach: Dr. Bu knew Viva's founders from 2015, years before the deal. They recognized natural synergy—Viva's structural biology and protein production capabilities complemented SYNthesis Med Chem's medicinal chemistry excellence.
- Strategic rationale over financial engineering: Rather than pursuing independent growth, SYNthesis Med Chem saw greater value in becoming part of an integrated platform that could serve global clients end-to-end while maintaining Melbourne-based expertise.
- Result: Australian medicinal chemistry excellence now embedded within a fully integrated Chinese CRO/CDMO platform, enabling Australian programs to access comprehensive capabilities while maintaining local scientific input.
The key lesson: the best deals come from relationships built over years, not months, with both parties clear on strategic rationale beyond just financial terms.
Navigating Geopolitical Complexity
Despite geopolitical tensions, complementary strengths create natural synergies: Australia's trusted regulatory environment provides validation pathways, while China's market scale and manufacturing infrastructure enable commercialization.
Dr. Marina Zhang brought essential perspective on the geopolitical dimension—a reality that can't be ignored but shouldn't paralyze action. While Canberra's foreign policy has moved toward pragmatic selective engagement with China, industry still lacks clear policy frameworks for when and how to pursue deeper collaboration. This uncertainty affects institutions wanting to pursue partnerships but unsure about government support or potential restrictions.
Rather than viewing geopolitics as a barrier, Dr. Zhang reframed the competitive dynamic around shared healthcare challenges: aging populations, pandemic preparedness, and chronic disease management affect both countries. She positioned Australia's middle-power status as a strategic asset. "If Australia exclusively aligns with the US in biotech innovation, it will lag behind other innovative countries," she noted. "Innovation isn't just zero to one—it's one to a hundred, and China has the scale and speed for that journey."
The evidence supports continued engagement: despite headlines about the US BIOSECURE Act and technology restrictions, licensing deals and M&A between Chinese biotechs and Western pharma have actually increased. Dr. Herath confirmed that Peter Mac operates with robust compliance frameworks while maintaining extensive Chinese sponsor relationships, and Maria highlighted university sophistication in managing these complexities. The panel's consensus was clear: transparent governance and strong IP strategies are essential for managing risks while capturing opportunities.
Moving from Dialogue to Action
The panel discussion concluded with a clear call to action for all stakeholders. For Australian biotechs and researchers: Begin exploring specific partnership opportunities in areas where your technology addresses unmet needs in Asian markets. Explore engaging with service providers to streamline drug development and leverage China’s supply chain capabilities. For investors: Consider how cross-border collaboration can create value and reduce development risks for portfolio companies. For universities: Develop institutional frameworks and support services that enable researchers and entrepreneurs to pursue China partnerships strategically. For policymakers: Work toward regulatory harmonization initiatives and bilateral innovation agreements that provide certainty for long-term collaboration.
As China's biotech ecosystem continues its rapid evolution toward global innovation leadership, proactive engagement from Australian institutions and companies is essential to capture opportunities and compete globally. Betty Zhangemphasized in closing the panel, "The opportunities are clear, the pathways are becoming better defined, and the time to act is now. Let's work together to turn today's insights into tomorrow's partnerships."
Strategic Geography: Where to Run What
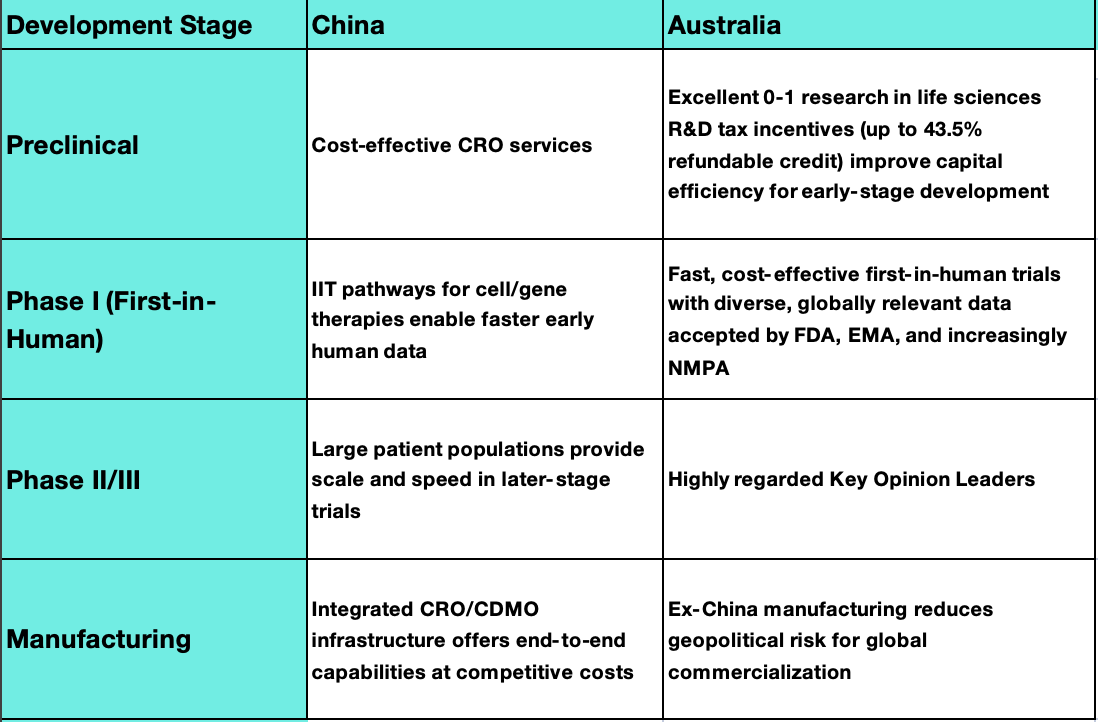
Key Takeaways: Making Partnerships Work
Choose integrated partners who provide services across the development value chain rather than coordinating multiple vendors.
Invest in protocol quality upfront—trial design quality often determines success more than the molecule itself.
Select CROs carefully through thorough due diligence on capabilities, quality systems, and track records.
Build local-local syndicates pairing Australian investors with Chinese or US funds to leverage local knowledge and networks.
Plan dual regulatory pathways early—think through TGA and NMPA strategies from the start; consider Hong Kong's Greater Bay Area Medical Connect as a bridge.
Start with pilot projects to test partnerships on smaller scope before major commitments, assessing quality, communication, and cultural fit.
Invest in relationships early through repeated face-to-face meetings and sustained engagement—the best deals come from relationships built over years.
Maintain flexible structures to preserve optionality for future funding rounds and partnerships in other geographies.
Looking Ahead
The panel established that successful collaboration requires moving beyond strategic alignment to concrete execution—selecting right partners, investing in quality from the start, and building trust through sustained engagement.
But questions remain: How are investors structuring deals? What's driving the out-licensing surge from Chinese biotechs? How do NewCos work? What role does Hong Kong play in capital flows?
These questions formed the basis for the investor fireside chat—explored in Part 3 of this series.
Next in this series: Part 3 examines the investment landscape, capital flows, and deal structures enabling cross-border biotech partnerships, featuring insights from Lilly Asia Ventures and Boson Ventures.
For more information about Australia-China bioinnovation initiatives, contact Boson Ventures at contact@boson.vc
Join Our Community Today!
Stay updated with our latest insights and news.
Explore Our Latest Insights
Stay updated with our recent articles.


.png)
